Now live! RTHM Direct – simplified medication access for Long COVID, ME/CFS and related conditions. Check it out
As RTHM continues to learn more about Long COVID, ME/CFS, and other common comorbidities, the presence of autoantibodies and how they interact with the body and bind to G-protein coupled receptors (GPCRs) has gained attention. These receptors play a vital role in heart rate regulation, nerve signaling, blood flow, and other processes within the body. Autoantibodies can disrupt this normal function, resulting in many of the symptoms those with Long COVID, ME/CFS, Dysautonomia, and more live with every day.
A Theory Behind Autoantibody Testing?
Infections, like the SARS-CoV-2 virus responsible for COVID-19, can often trigger an immune response within the body that, in some cases, is exaggerated and can lead to chronic inflammation that affects various organs and areas of the body. This exaggerated response to the initial infection can also trigger the production of autoantibodies which then turn on GPCRs and other cells in the body, triggering new or continued symptoms, such as seen in Long COVID.
GPCR+ autoantibody testing is grounded in research that links autoantibodies with receptor dysfunction that may be responsible for many of the symptoms seen with complex illnesses like Long COVID, ME/CFS, and Dysautonomia. (Wallukat et al. 2021) (Li et al. 2014) (Loebel et al. 2016)
Skiba MA, Kruse AC. Autoantibodies as Endogenous Modulators of GPCR Signaling. Trends Pharmacol Sci. 2021 Mar;42(3):135-150. doi: 10.1016/j.tips.2020.11.013. Epub 2020 Dec 24. PMID: 33358695; PMCID: PMC7880908.
What are Antibodies?
Antibodies, also known as immunoglobulins, are protective proteins produced by your immune system. They are designed to bind specifically and neutralize invaders such as bacteria, fungi, viruses, and toxins, which are known as antigens.
Antibodies are produced by B cells, a type of white blood cell. When an antigen comes into contact with a B cell, it triggers the B cell to divide and clone itself. These cloned B cells, also known as plasma cells, release millions of antibodies into your bloodstream and lymph system.
Each antibody is a Y-shaped molecule made up of two heavy chains and two light chains. The tips of the “Y” shape form the antigen-binding sites, which are highly variable and allow the antibody to bind specifically to its corresponding antigen.
Antibodies play a crucial role in the immune system by neutralizing pathogens or marking them for destruction by other immune cells. They can also bind to antigens on the surface of harmful cells, signaling the immune system to destroy those cells.
What are Autoantibodies?
Autoantibodies are a type of antibody. However, unlike typical antibodies that target foreign substances in the body (like bacteria and viruses), autoantibodies target the body’s own cells, tissues, or other substances naturally present in the body.
This can happen when the immune system mistakenly identifies these substances as foreign. The production of autoantibodies can lead to autoimmune diseases, where the immune system attacks the body’s own cells. Examples of such diseases include type 1 diabetes, rheumatoid arthritis, and lupus among others.
Skiba MA, Kruse AC. Autoantibodies as Endogenous Modulators of GPCR Signaling. Trends Pharmacol Sci. 2021 Mar;42(3):135-150. doi: 10.1016/j.tips.2020.11.013. Epub 2020 Dec 24. PMID: 33358695; PMCID: PMC7880908.
What are GPCRs?
G-protein-coupled receptors (GPCRs) are a large family of transmembrane proteins that play a crucial role in many biological processes and are involved in a variety of diseases. Transmembrane proteins are proteins that span across cell membranes; they receive signals external from the cell and transmit them internally for further biological processes. They are the largest family of transmembrane proteins in the human genome with more than 800 unique GPCRs.
These receptors are coupled to intracellular GTP-binding proteins (G-proteins). Once activated, G-proteins trigger the production of a variety of second messengers (e.g. cyclic AMP [cAMP], inositol triphosphate [IP3], diacylglycerol [DAG], etc.) helping to regulate a number of body functions ranging from sensation to growth to hormone release.
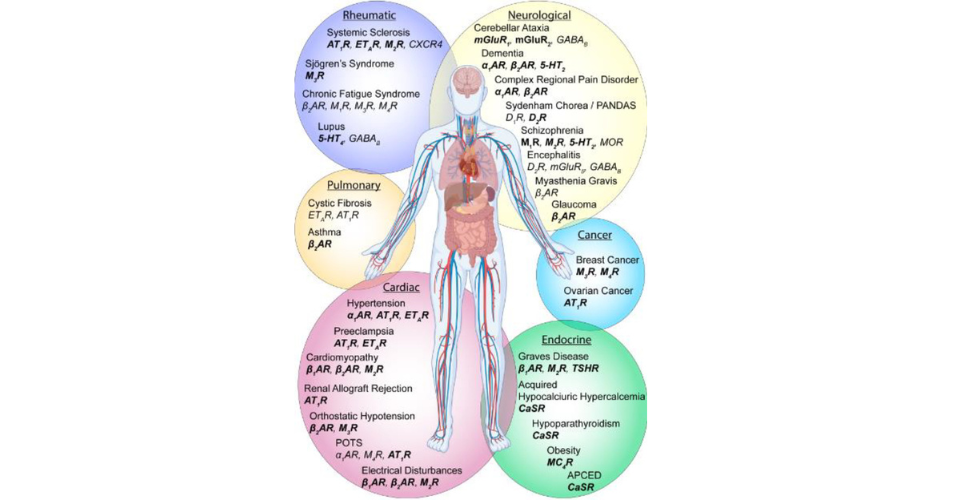
GPCRs (G-protein coupled receptors) play a significant role in connecting to chronic diseases. They are involved in various physiological processes and are considered optimal regulators of complex biological systems. GPCRs are implicated in monogenic diseases, genetic variants, and as drug targets. Antibodies against GPCRs serve as biomarkers for various diseases and can modulate autoimmune and non-autoimmune diseases. GPCRs also play a role in the regulation of inflammation and are associated with diseases such as inflammatory bowel disease. Understanding the function of GPCRs can lead to the identification of novel therapeutic strategies for chronic diseases. (Insel et al. 2007) (Leysen et al. 2022) (Riemekasten et al. 2021) (Melhem et al. 2019) (Cook and Mansuy-Aubert 2022) (Schukur and Fussenegger 2017) (Saifeddine et al. 2016) (Naviaux 2019) (Liu and Ji 2014)
How Do Autoantibodies Affect GPCRs?
In the context of autoantibodies, research suggests that autoantibodies can target GPCRs. This can lead to either activation or inhibition of the receptor’s function, depending on the nature of the autoantibody. This interaction can contribute to the pathogenesis of various diseases.
For example, in some cases of autoimmune diseases like Graves’ disease (an autoimmune thyroid disorder), autoantibodies are produced that can bind to the thyroid-stimulating hormone receptor (a type of GPCR) on the thyroid gland. This can lead to overstimulation of the thyroid gland, resulting in hyperthyroidism.
In other cases, such as certain types of cardiac conditions, autoantibodies can target beta-adrenergic receptors (another type of GPCR), potentially leading to alterations in heart function.
In cases of chronic conditions, such as POTS, ME/CFS, and Long COVID, some studies show the potential association of autoantibodies, though more research is needed to understand their role better. For example, autoantibodies targeting adrenergic receptors or ganglionic acetylcholine receptors have been detected in a subset of POTS patients. Similarly, in ME/CFS, there have been reports of autoantibodies targeting various cellular components, such as mitochondria and ion channels. However, the presence of these autoantibodies is not consistently observed in all ME/CFS patients. Regarding Long COVID, some studies have reported the presence of autoantibodies targeting specific proteins, such as angiotensin-converting enzyme 2 (ACE2) or immune system components. (Healthline 2023) (Sapkota and Nune 2022) (Davis et al. 2023) (Astin et al. 2023)
However, the exact mechanisms and implications of these interactions are complex and can vary widely depending on the specific autoantibody and GPCR involved, and are still an active area of research.
Good Video – Post-COVID ME/CFS and the Role of GPCR Antibodies

Common Antibodies Included in a GPCR+ Autoantibody Panel?
Angiotensin-converting enzyme 2 (ACE2) autoantibody
ACE2 primarily serves as a counter-regulatory enzyme to the renin-angiotensin system (RAS), converting angiotensin II to angiotensin-(1-7), promoting vasodilation and reducing inflammation and fibrosis. Additionally, ACE2 is recognized as the primary receptor for the SARS-CoV-2 virus, the causative agent of COVID-19. Click HERE for more information.
Alpha 1 adrenergic receptor (A1AR) autoantibody
The α1-AR is crucial in mediating vasoconstriction in response to catecholamines, influencing blood pressure and vascular tone. Click HERE for more information.
Angiotensin II receptor 1 (AT1R) autoantibody
The AT1-R mediates most of the known actions of angiotensin II, including vasoconstriction, aldosterone synthesis, and renal sodium retention, playing a central role in blood pressure regulation and electrolyte balance. Click HERE for more information.
Beta 1 adrenergic receptor (B1AR) autoantibody
The β1-AR plays a crucial role in mediating the positive inotropic (strength of contraction) and chronotropic (rate of contraction) effects on the heart in response to catecholamines, especially norepinephrine and epinephrine. Click HERE for more information.
Beta 2 adrenergic receptor (B2AR) autoantibody
The β2-AR primarily mediates the relaxation of smooth muscles, especially in the bronchioles of the lungs, making it a crucial target for asthma and COPD (chronic obstructive pulmonary disease) treatments. Click HERE for more information.
Endothelin A receptor (ETAR) autoantibody
The ETAR primarily mediates vasoconstriction and cell proliferation in response to endothelin 1, playing a significant role in vascular tone and contributing to various cardiovascular and renal diseases. Click HERE for more information.
MAS1 receptor (MASR) autoantibody
MAS-R is activated by angiotensin-(1-7) and opposes the actions of the classic renin-angiotensin system (RAAS), promoting vasodilation, anti-inflammatory, anti-fibrotic, and anti-thrombotic effects, thus playing a protective role in cardiovascular and renal systems. Click HERE for more information.
Muscarinic cholinergic receptor 1 (M1R) autoantibody
M1-R is predominantly expressed in the brain and plays a crucial role in modulating neurotransmission, particularly in cognitive processes, learning, and memory. Its activation enhances neuronal excitability and is implicated in various central nervous system functions. Click HERE for more information.
Muscarinic cholinergic receptor 2 (M2R) autoantibody
M2-R is primarily located in the heart, where its activation leads to a decrease in heart rate and cardiac contractility by inhibiting adenylate cyclase activity, thereby acting as a counter to the sympathetic nervous system’s stimulatory effects on the heart. Click HERE for more information.
Muscarinic cholinergic receptor 3 (M3R) autoantibody
M3-R is widely expressed in various tissues, with prominent roles in mediating smooth muscle contraction (e.g., bronchial and gastrointestinal muscles) and stimulating glandular secretion, especially in the salivary and lacrimal glands. Activation of M3-R leads to increased intracellular calcium via the IP3 pathway. Click HERE for more information.
While RTHM is no longer offering direct testing, CellTrend will be able to provide information on how you can test today. Visit CellTrend

Get updates
Join our mailing list